Silicon nitride is a ceramic chemical compound comprised of silicon and nitrogen and is widely used across many industries. Silicon nitride offers interesting mechanical and thermal properties, which can be tailored for use in many specific high-temperature and wear-resistant applications, as well as its use as an insulating and chemical barrier in integrated circuits.1 However, to gain a comprehensive understanding of the behavior and properties of silicon nitride, it is necessary to understand its atomic structure. In this blog post, we will explore the atomic structure of silicon nitride and how this influences its properties.
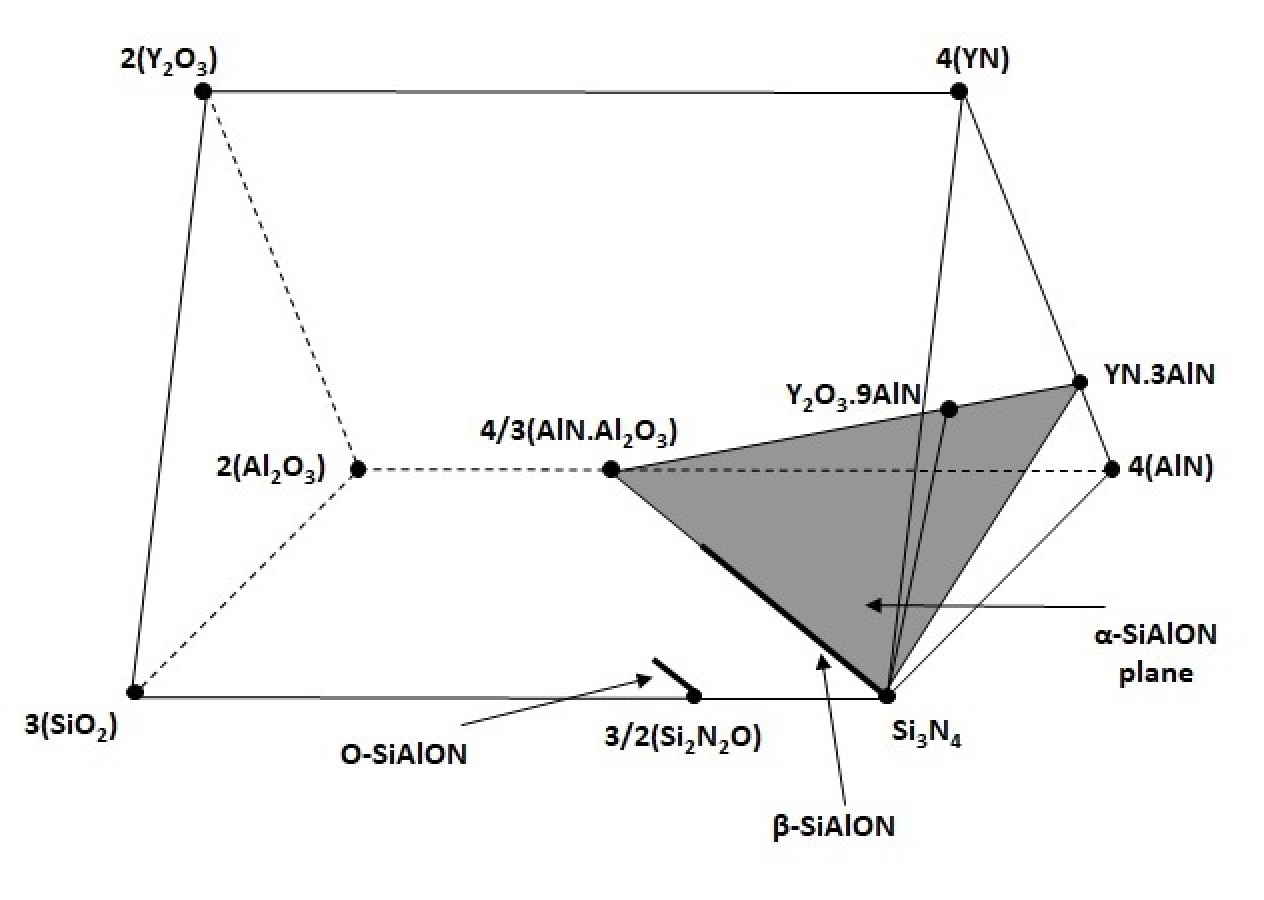
Phase diagram for the Y-Si-Al-O-N system.
A Quick Guide to Silicon Nitride
The fundamental structural unit of silicon nitride is the SiN4 tetrahedron, which is similar to the SiO4 structural units found in silicates. The SiN4 tetrahedra are connected by sharing corners to form a rigid three-dimensional framework. The Si-N bonds in this framework are short and strong covalent bonds, and contribute to many of the important properties of Si3N4, such as its high strength, high hardness, and excellent resistance to corrosion, thermal shock, and wear.
Silicon nitride (Si3N4) can be modified by the addition of alumina (Al2O3) to form Si-Al-O-N without changing the structure. Sialon, or silicon aluminum oxynitrides, are a well established family of ‘alloyed-ceramics’ that are produced by substituting up to two-thirds of the Si with Al, provided the equivalent concentration of N is replaced with O.2 SiAlONs exist in three basic forms, each of which is isostructural with one of the two common forms of Si3N4, beta (β) and alpha (α), as well as with silicon oxynitride.
The substitution of Al for Si creates a solid solution with a more open crystal structure with larger spaces that can enhance the diffusion and transport properties of the material and which forms a liquid at lower temperatures with oxide additives such as Y2O3, allowing sialon to be densified by pressureless sintering like a traditional ceramic and which can reduce porosity in the sintered material and improve its strength, hardness, and fracture toughness.
The principles of alloying and exchanging Al for Si and O for N in SiAlONs is similar to the way that brass is made by replacing some of the copper (Cu) atoms in pure copper (Cu) with zinc (Zn) atoms. This results in a stronger and better alloy than the original metal. Similarly, the substitution of Al and O in SiAlONs can result in an alloy that has superior properties to the original silicon nitride.

High temperature and wear resistant Syalon 101 components. Image Credit: International Syalons (Newcastle) Ltd.
International Syalons and Silicon Nitride
International Syalons is the leading supplier of sialon and silicon nitride-based materials in the UK but with an international client base. We are committed to providing first-class ceramic solutions for your industrial materials requirements and continue to develop cutting-edge composites, materials, and fabrication processes to provide the best solutions for our clients. Our silicon nitride and sialon ceramics offer high levels of corrosion, thermal and wear resistance and are suitable for ceramic tubes, foundry products and many other applications.
To learn more about silicon nitride and its applications, contact a member of International Syalons today.

